Background: Interaction between CXCL12 (SDF-1) and its receptor, CXCR4, plays a critical role in the retention of CD34+ hematopoietic stem and progenitor cells (HSPCs) in the bone marrow. Motixafortide (motix) is a novel selective CXCR4 antagonist with high affinity for CXCR4 (IC 50 0.42 - 4.5nM), slow dissociation (Kd = 3.38e-5 s-1), and high plasma protein binding (>99%). In the Phase 3 GENESIS trial (NCT03246529), motix added to G-CSF (G) resulted in significantly higher mobilization of HSPCs in patients with multiple myeloma (MM) prior to autologous stem cell transplantation, with 88.8% of patients collecting ≥6x10 6 CD34+ cells/kg (local lab) in 1 leukapheresis procedure (LP) 10-14 hours following a single dose of motix (1.25mg/kg). Of note, GENESIS trial patients requiring additional LPs to meet HSPC mobilization goals underwent a second LP at 34-38 hours post-motix, with a total of 92.5% of patients meeting their collection goal. Here, we report in-vitro receptor occupancy, as well as clinical pharmacokinetics (PK) and pharmacodynamics (PD) of peripheral blood (PB) mobilization of CD34+ cells/ μL in healthy volunteers (HV) and patients with MM following motix administration.
Methods: CXCR4 receptor occupancy and dissociation rate were assessed by incubating increasing doses of motix (3-500nM) with Jurkat cells in vitro followed by washing and incubation 0-72 hours later with either an anti-CXCR4 12G5 fluorophore-conjugated monoclonal antibody (mAb) (competes with motix for binding to CXCR4) or an anti-CXCR4 1D9 fluorophore-conjugated mAb (binds to CXCR4 but does not compete with motix) followed by flow cytometry. In HV (NCT05293171), PB samples were collected following a single subcutaneous (SC) administration of motix (1.25mg/kg). PK timepoints were pre-dose and at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, 12, 16, and 24 hours post-motix; and PD timepoints were pre-dose and at 0.75, 2, 4, 8, 12, 16, and 24 hours post-motix. In patients with MM, PB samples were collected following SC motix (1.25mg/kg) added to G. PK timepoints were pre-motix (post-G) and 0.25, 0.5, 1, 2, 4, 8, and 12 hours post- motix; and PD timepoints were pre-motix (post-G) and 12 hours post-motix.
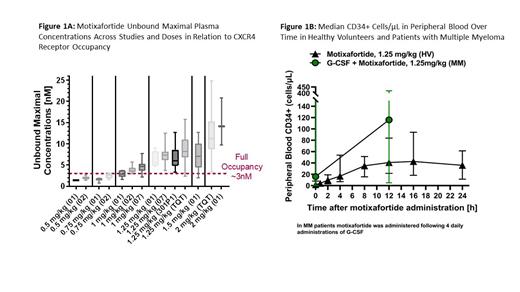
Results: In vitro studies demonstrated complete CXCR4 receptor occupancy at 3 nM (6.48 ng/mL) of motix, with increasing concentrations resulting in longer receptor occupancy of >72 hours. Due to high protein binding, a minimum total plasma concentration of 800 ng/mL is required to achieve a free fraction of 3nM. This level was achieved following administration with doses ≥ 1.0 mg/kg (Fig 1A). PK analysis in HVs and in patients with MM dosed with 1.25 mg/kg demonstrated that motix was rapidly absorbed (maximum concentration within 0.25-1 hours). Mean maximal plasma concentrations were 2245 ± 791 ng/mL (1040nM) in HVs and 1800 ± 695 ng/mL (834nM) in patients with MM. Notably, the half-life of motix was 1-3 hours. However, despite the short half-life, the high CXCR4 receptor affinity and slow dissociation rate of motix led to an extended PD effect. In HVs who received motix as monotherapy, median PB CD34+ counts increased from baseline of 1.6 CD34+ cells/µl to 17 CD34+ cells/ μL within 4 hours post-motix, reached maximal levels of 43 CD34+ cells/μL at 16 hours post-motix and remained elevated at 36 CD34+ cells/μL at the last observation time point 24 hours post-motix (Fig 1B). In MM patients who received motixafortide after 4 doses of G-CSF, there was a 6.6 fold increase in median PB CD34+ cell counts, from 15.8 cells/μL (post-G-CSF, pre-motix) to 116.0 CD34+ cells/μL at 12 hours post-motix (Fig 1B).
Conclusions:
In vitro studies demonstrated complete receptor occupancy by motix starting at a concentration of 3nM. Increasing concentrations resulted in longer duration of receptor occupancy. Mobilization of CD34+ cells to PB was observed within 2 hours after motix injection (1.25mg/kg), peaked at 12-16 hours, and remained elevated (>20 CD34+ cells/μL) for >24 hours post-motix. These PK/PD characteristics of motix result in rapid and robust PB CD34+ HSPC mobilization (median 116.0 CD34+ cells/μL); enabling 88.8% of patients to collect ≥6x10 6 CD34+ cells/kg in 1 LP with 1 dose of motix added to G. Furthermore, the extended PD effect of motix supports a flexible administration window that allows for LP to be performed > 24 hours post-motix administration.
Disclosures
Crees:BioLineRx, Ltd.: Other: Advisory Board, Research Funding. Stockerl-Goldstein:Celgene: Consultancy. Sorani:Biolinerx Ltd.: Current Employment, Current equity holder in publicly-traded company. Ickowicz:BiolineRx Ltd: Current Employment. Darvish:BiolineRx Ltd: Current Employment. DiPersio:Sun Pharma Ltd.: Membership on an entity's Board of Directors or advisory committees; hC Bioscience, Inc.: Membership on an entity's Board of Directors or advisory committees; Magenta: Other: Ownership Investment, Patents & Royalties; Vertex: Consultancy; WUGEN: Other: Ownership Investment, Patents & Royalties; Washington University: Current Employment; Macrogenics: Consultancy, Research Funding; NeoImmune Tech: Consultancy; Incyte: Consultancy, Research Funding; BiolineRx Ltd: Consultancy, Research Funding; RiverVest Venture Partners: Membership on an entity's Board of Directors or advisory committees; Amphivena Therapeutics: Research Funding.